Olive Oil Industry Moves to Oppose Proposed FDA Front-of-Pack Label
Stakeholders in the olive oil industry are opposing the FDA’s proposed front-of-pack nutrition label, arguing that it will confuse consumers and unfairly categorize olive oil. The FDA’s proposed label would categorize olive oil as medium in saturated fat content, which has led to criticism and calls for a different approach to providing nutrition information on food packaging.
Olive oil industry stakeholders are pushing back against the United States Food and Drug Administration’s (FDA) proposed front-of-pack nutrition label.
The FDA, which announced the proposed rule in January, said the label would take the form of a nutrition information box, providing consumers “readily visible information about a food’s saturated fat, sodium and added sugars content — three nutrients directly linked with chronic diseases when consumed in excess.”
See Also:Nutri-Score Outperforms Nutrinform Battery with Portuguese ConsumersThe nutrition information box will be divided into three columns, detailing the nutrient, the percent of daily value, and whether this value is low, medium, or high.
Food defined as “low” in a specific nutrient will contain less than five percent of its daily value. “Medium” foods will have between six and 19 percent of the daily value of the nutrient, and foods with more than 20 percent of the daily value will be marked as “high.”
Olive oil, a serving of which contains about ten percent of the daily value of saturated fat, would fall into the medium category, along with popular brands of ultra-processed snack foods, such as Doritos and Pringles. Meanwhile, other ultra-processed foods, including Oreos and Funyuns, would qualify as “low” in saturated fat.
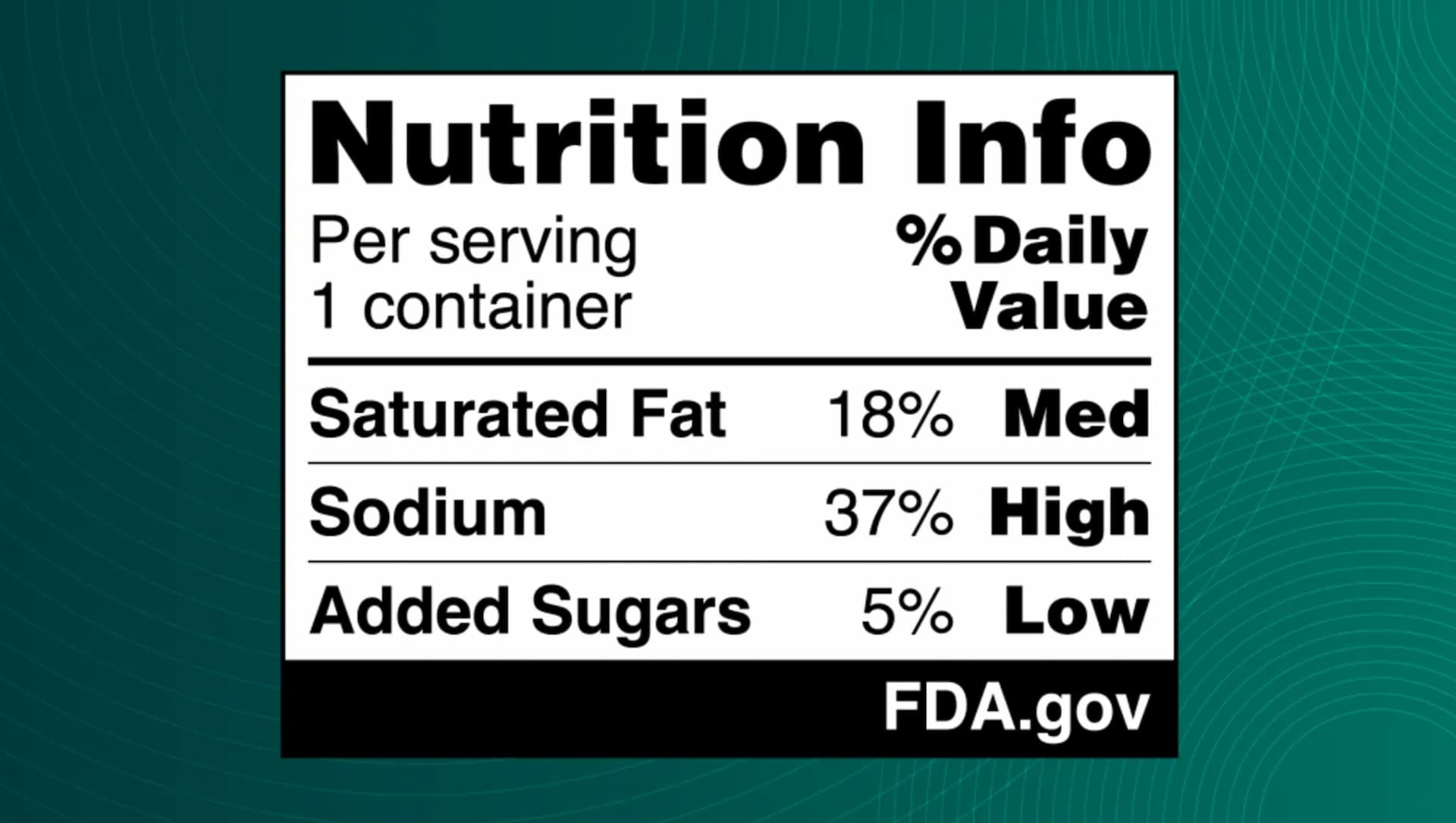
A sample of the FDA’s proposed front-of-pack label (Photo: FDA)
Joseph R. Profaci, the executive director of the North American Olive Oil Association, whose 60 members represent an estimated 70 percent of United States olive oil sales, lamented the decision and is calling on the FDA to withdraw the proposed front-of-pack label.
“The NAOOA opposes the proposed FOP [front-of-pack label] rule as written because it will send confusing and conflicting messages to consumers with respect to olive oil labels,” he wrote in a public comment to the FDA, which was seen by Olive Oil Times.
While the NAOOA stated that it agreed in principle to providing nutrition information to help consumers make healthy decisions, Profaci warned that categorizing foods based on a single nutrient was a step backward.
Instead, he praised a previous initiative by the FDA that allowed health claims on foods to help consumers follow a healthy dietary pattern based on current nutrition science.
The FDA cited olive oil as an example of a food that was previously excluded from receiving a “healthy” claim but now meets the new criteria, as its saturated fat content is below 20 percent of the daily value.
“This is why the NAOOA was disappointed to see that under the proposed FOP rule, FDA has reverted to the discredited one-size-fits-all approach of the 1990s by requiring an across-the-board definition of what constitutes low, medium and high content of the reference nutrients,” Profaci wrote.
The NAOOA further criticized the application of the rule to fats and oils, saying it made no sense for olive oil producers and sellers to list sugar or sodium content. However, the NAOOA added that an exemption for single-ingredient foods would also be an unsatisfactory outcome.
“The FOP box should instead only provide information on the specific nutrients that are relevant to the food group to which a food belongs,” Profaci wrote.
“A consumer looking to purchase a cooking oil to include as part of their diet would benefit at the point of decision if they could compare the relevant nutrient content among the available options, e.g. olive oil or corn oil — not by comparing the nutrient content of the cooking oil to a food in a different group, such as lemonade,” he added.
The NAOOA warned that the “healthy” claim currently permitted on labels, combined with a “medium” rating for saturated fat content, may confuse consumers.
Instead, the NAOOA recommended having different front-of-pack labels for specific food groups, such as one for fats and oils that lists the content of saturated fat, trans fat, cholesterol, and oleic acid to help consumers make more informed decisions, or scrapping the idea altogether and coming up with a different type of label.
Along with the NAOOA, the California Olive Oil Council and American Olive Oil Producer Association are expected to submit comments in opposition to the labeling scheme. To date, the FDA has received over 11,000 comments on the labeling scheme.









